Uranium (U): A Pillar in Modern Science and Technology
Uranium, symbol U, is a pivotal element in the modern periodic table known for its significant role in energy production and technology. This comprehensive guide delves into the history of Uranium, its scientific characteristics, and its wide-ranging applications in various fields. Uranium’s unique properties make it indispensable in nuclear power generation and have applications spanning from military uses to medical imaging technologies, emphasizing its crucial role in contemporary technological advancements.
Discovery of Uranium

Uranium was discovered in 1789 by the German chemist Martin Heinrich Klaproth. He discovered it in the mineral called pitchblende, which he named after the planet Uranus, discovered just eight years earlier. Klaproth was able to isolate an oxide of uranium but not the pure metal itself, which was later isolated by Eugène-Melchior Péligot in 1841. Uranium's early use was primarily as a colorant for glass and ceramics, showcasing vibrant yellow and green colors. The discovery of Uranium opened up new pathways in science, particularly in the fields of chemistry and physics, leading to groundbreaking developments in energy that have had lasting impacts on global power strategies and medical research.
Uranium in the Periodic Table
Located under the actinide series, Uranium holds the atomic number 92. It is the heaviest naturally occurring element on Earth, commonly found in low concentrations in soil, rock, and water. Uranium is unique among the elements for its highly unstable and radioactive properties, which are central to its modern applications. The element occupies a critical position in the periodic table, bridging stable elements and those only artificially synthesized.
Physical and Chemical Properties of Pure Uranium

Pure uranium, a dense and silvery metal, stands out due to its notable density, which is around 70% higher than that of lead. It is weakly radioactive and has several isotopes, with Uranium-238 being the most common in nature, comprising over 99% of naturally occurring uranium. Pure uranium is also chemically reactive, easily forming compounds with most non-metal elements. Additionally, its oxides are known to be soluble in both acidic and alkaline water. This reactivity plays a significant role in pure uranium's utilization in various industrial applications and in its interaction with environmental factors.
Mining the Element Uranium

Uranium mining is a complex process that often involves extracting the element from ore deposits in the Earth's crust. This heavy metal frequently co-occurs with other minerals and elements such as radium, vanadium, and thorium. These associated elements can sometimes be recovered during the uranium milling and refining process. Extracting uranium not only provides fuel for nuclear power but also yields these valuable byproducts, which have various industrial and medical applications.
Significant uranium mining regions include the Athabasca Basin in Canada, known for having some of the highest-grade uranium deposits in the world, and the Kazakhstan region, which is one of the largest global producers. In the United States, uranium is primarily mined in states such as Wyoming and New Mexico. Each of these areas features unique geological formations that make them rich in uranium and conducive to mining operations.
One of the largest mines is the Rossing Mine in Erongo, Namibia.
Applications of Uranium in Technology
Uranium Nuclear Energy
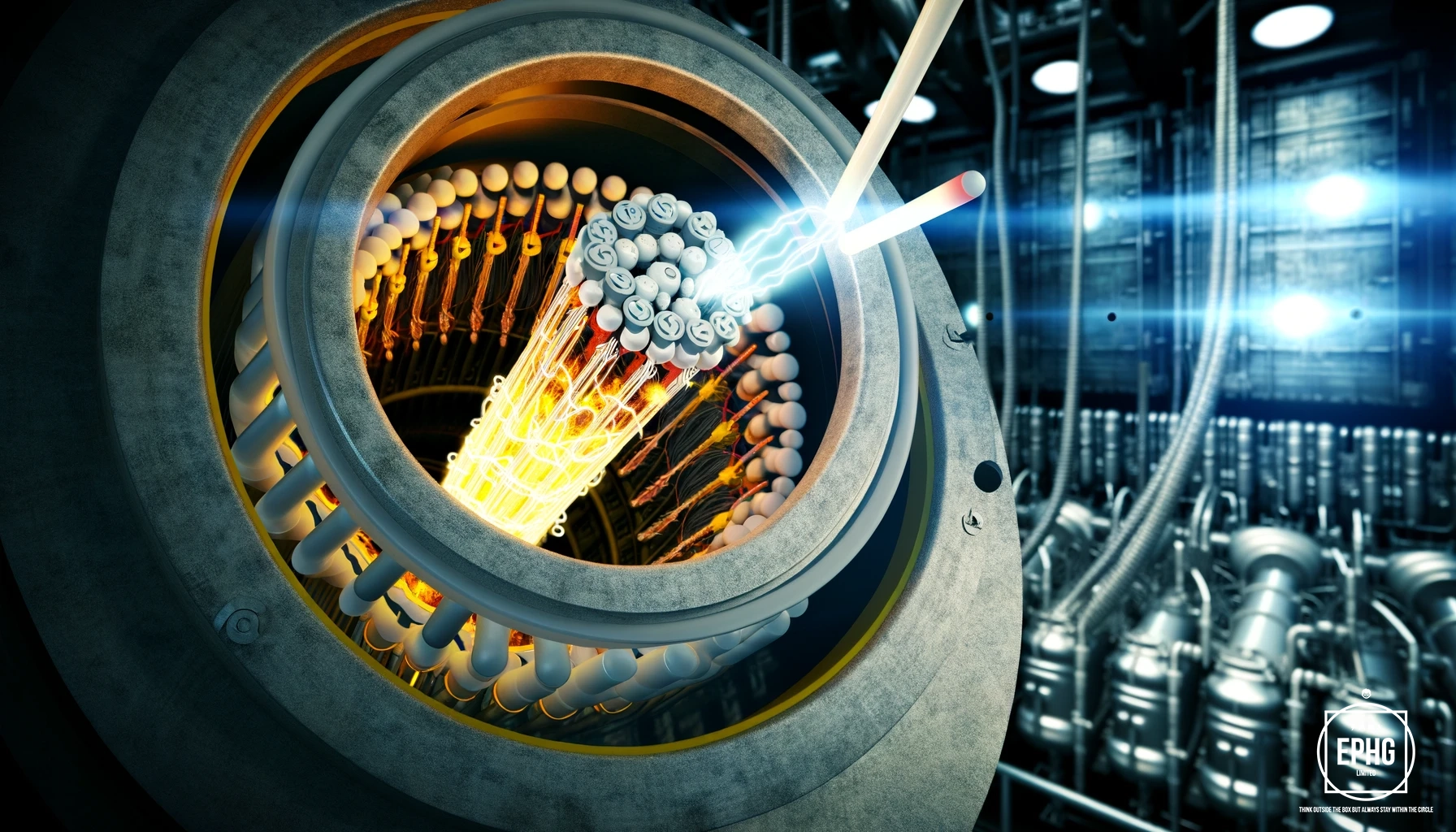
The most notable use of Uranium is in nuclear power generation. Uranium-235, an isotope found naturally in uranium ore, is key for sustaining nuclear fission. It is primarily used as a fuel in nuclear reactors to produce heat, which in turn generates electricity. The process of nuclear fission involves the splitting of Uranium-235 nuclei, releasing a substantial amount of energy. This energy is harnessed to heat water into steam, which then drives turbines to produce electricity. Compared to traditional fossil fuels like coal and gas, the energy produced by nuclear fission from uranium is significantly cleaner, contributing a substantial percentage of the world's carbon-free electricity and helping to mitigate climate change.
Uranium Military Uses
Depleted Uranium, a by-product of enriching Uranium-235, is used in military applications, particularly in armor-piercing projectiles. Its high density makes it effective at penetrating armored targets. However, its use remains controversial due to potential health and environmental risks.
Space Exploration using Uranium

Uranium's potential applications extend far beyond terrestrial uses, reaching into the realm of space exploration. Due to its high density and radioactive properties, uranium can be harnessed to provide long-lasting power sources for spacecraft and extraterrestrial bases. This makes it a prime candidate for future deep space missions. Additionally, the energy efficiency and reliability of uranium could support life support systems and scientific instruments in harsh, off-world environments, enhancing the sustainability of long-duration space endeavors.
Medicine from Uranium
Radiopharmaceuticals containing uranium isotopes are utilized in diagnostic imaging. These compounds help in tracing and imaging organs, providing valuable diagnostic information. Research continues into expanding the use of uranium isotopes in targeted cancer treatments.
Challenges and the Future of Uranium

While Uranium plays a crucial role in modern energy infrastructure, it poses significant challenges, including radioactive waste management, environmental concerns, and geopolitical tensions over its supply and regulation. Advancements in reactor technology and uranium recycling promise to enhance the efficiency and safety of nuclear power, potentially leading to a renaissance in uranium-based energy systems.
The refined image vividly depicts a futuristic starship, designed for long-duration space travel in the year 2100, with a focus on advanced Uranium Energy propulsion. The starship is sleek and aerodynamic, showcasing a prominent, vibrant glow from its uranium-powered propulsion system, which serves as the primary energy source. The enhanced technological details underscore its cutting-edge design, suitable for interstellar journeys. Set against a backdrop of distant stars and colorful nebulae, the image highlights the starship's capability to navigate the depths of space, representing the pinnacle of future space exploration technology.













