The Comprehensive Guide to Platinum (Pt): Properties, Science, and Applications
Introduction to Platinum
Platinum, with the symbol Pt and atomic number 78, is a precious metal renowned for its lustrous sheen and myriad applications. This rare, silvery-white metal is not only a symbol of luxury but also a pivotal component in various technological innovations.
Discovery of Platinum

Platinum was first documented by European scientists in the 16th century after being brought to Europe from the Americas, where indigenous peoples had already been using it. However, it was not until Antonio de Ulloa, a Spanish scientist and explorer, published his findings in 1748 that platinum began to be seriously investigated by the European scientific community. His detailed description of this intriguing metal captured the attention of many, though its rarity and the challenge of melting it—due to its high melting point—initially limited its practical applications. This precious metal was often considered merely a curiosity in the scientific world until methods for its processing were developed in the 19th century.
Pure Platinum

Pure platinum is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Renowned for its physical properties, including its ability to resist corrosion even at high temperatures, platinum is often used in its pure form in a variety of industrial applications. These applications include jewelry making, where its luster and hypoallergenic nature are highly valued. Additionally, because it does not oxidize and is very stable, pure platinum is also utilized in medical implants and dental work. Its remarkable resistance to wear and tarnish makes it an ideal choice for fine jewelry and durable medical devices.
Platinum in the Periodic Table
As a member of the transition metals in the periodic table, platinum is known for its excellent corrosion resistance, remarkable ductility, and great stability. It plays a crucial role in understanding the behavior of d-block elements, particularly in terms of electron configuration and oxidation states.
For more detailed insights into the elements, visit our periodic table section.
Scientific and Technological Applications of Platinum
Platinum's applications are extensive and varied. In technology, it is used in the production of catalytic converters in vehicles, which help reduce harmful emissions. In medicine, platinum compounds are used in chemotherapy drugs to combat cancer. Furthermore, platinum is crucial in the manufacturing of electronic devices, dental equipment, and laboratory instruments.
In the field of energy, platinum plays a significant role in the development of fuel cells, which convert chemical energy into electricity. Its ability to facilitate chemical reactions without undergoing permanent changes makes it an ideal catalyst for these cells.
The Role of Platinum in Modern Industries

Platinum's impact extends beyond just technological applications. It is highly valued in the jewelry industry for its purity, non-reactive nature, and aesthetic appeal. Additionally, due to its rarity and intrinsic value, platinum is a popular investment commodity, often found in bullion coin and investment bar forms.
In the automotive industry, platinum-based catalysts are critical for meeting stringent emission standards. Similarly, in the petroleum refining industry, it is used to improve the efficiency of crude oil refining processes.
Platinum Production and Mining Sources
Platinum is primarily extracted through mining operations focused on Platinum Group Metals (PGMs), which include six closely related metals: platinum, palladium, rhodium, ruthenium, iridium, and osmium. These metals often occur together in nature and are found in ore deposits alongside gold and base metals such as nickel and copper. The major mines producing platinum include the Unki Mine in Zimbabwe, the Norilsk-Talnakh mines in Russia, and the Stillwater mine in the USA.
The extraction process involves crushing and milling the ore, followed by flotation to concentrate the PGMs. Subsequent smelting and chemical processes refine the metals to produce pure platinum and its sister metals. This complex extraction and refining process reflects the rarity and value of platinum and other PGMs.
Current Uses of Platinum
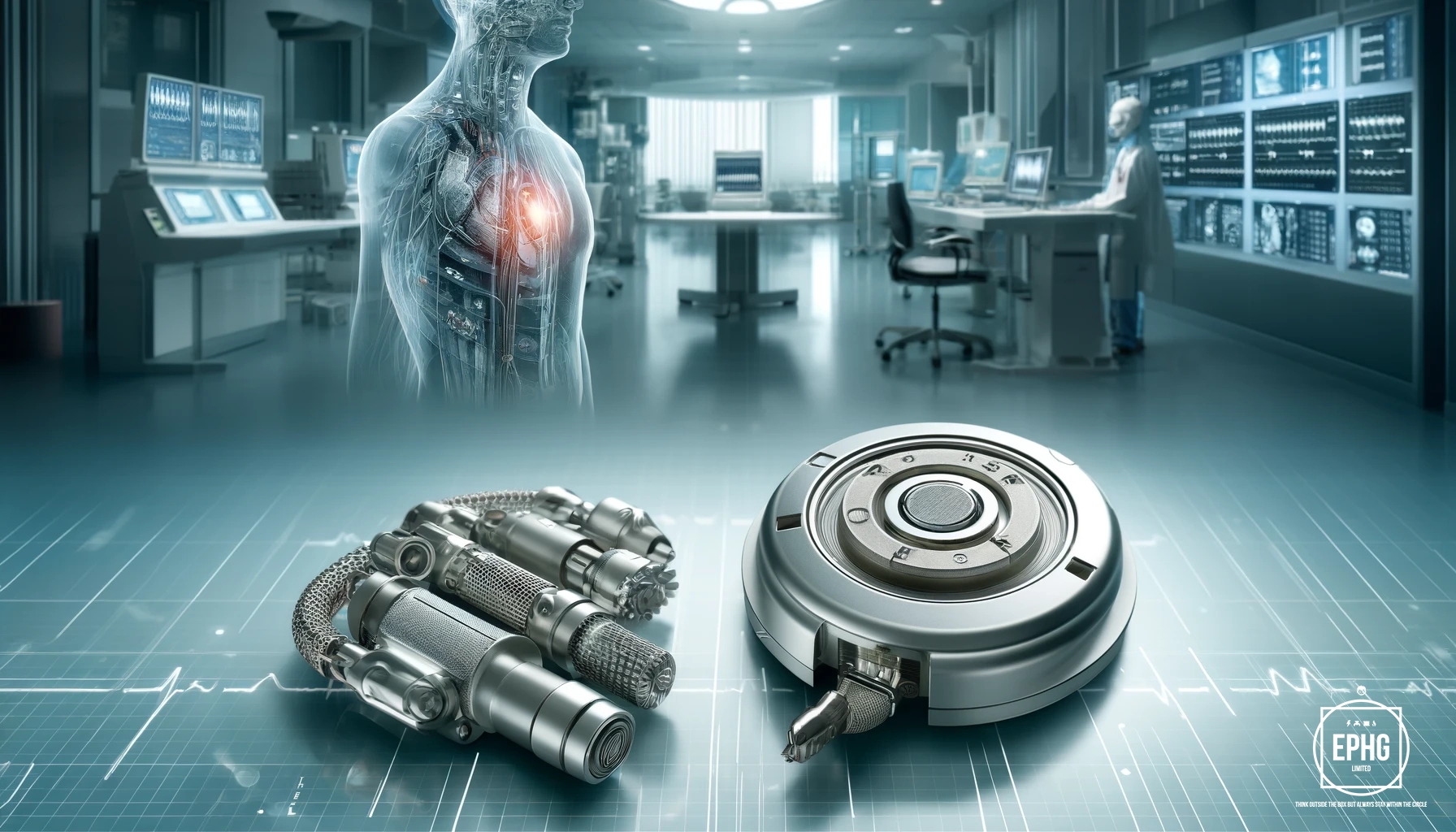
Today, platinum's uses are incredibly diverse, impacting many facets of modern technology and industry. It is essential in the automotive industry for the production of catalytic converters, which reduce harmful emissions. In medicine, platinum's biocompatibility makes it ideal for medical devices such as pacemakers and implantable defibrillators. Platinum's high resistance to heat and corrosion also makes it valuable in the manufacture of high-temperature equipment.
Additionally, platinum plays a critical role in various industrial applications, including as a catalyst in the chemical industry for the production of silicone and other materials, and in petroleum refining. Its rarity and aesthetic qualities continue to make it a popular choice in jewelry making.
The Future of Platinum

The future potential of platinum in science and technology is vast. Research is ongoing into its applications in hydrogen fuel cells, which could revolutionize the clean energy sector. Platinum may also play a critical role in next-generation computer hardware for faster and more efficient processors. Moreover, the exploration of platinum nanoparticles is expected to drive advancements in catalysis and sensors, pushing the boundaries of what is possible in technology and medicine.
As the world moves towards more sustainable practices, the demand for platinum is expected to increase, not only for its current applications but also for its role in emerging technologies that contribute to energy efficiency and environmental preservation.















