Iodine: An Indispensable Element in Health and Science
Discovery of Iodine
In 1811, the element iodine was first identified by French chemist Bernard Courtois during an experiment unrelated to his primary research goals. While processing seaweed ash to produce saltpeter for gunpowder, Courtois observed an unexpected phenomenon.

Upon adding sulfuric acid to the ash, he noticed striking violet vapors that were released. Intrigued by these vibrant emissions, he investigated further and eventually isolated the element responsible. He named this new element 'iodine', derived from the Greek word 'iodes', meaning violet, reflecting the color of its vapors when heated. This accidental discovery by Courtois not only added a vital element to the periodic table but also marked a significant advancement in the field of chemistry.
Iodine's Position in the Periodic Table
Iodine holds the atomic number 53 in the periodic table. It is a member of the halogen group, known for their reactivity and distinctive non-metallic properties. Iodine is less reactive compared to chlorine and fluorine but shows significant reactivity and a unique set of properties that make it essential in various industries.
Physical and Chemical Properties of Iodine and it's purest form

Iodine, in its purest form, presents as a dark gray or purple-black crystalline solid at room temperature. Known for its dramatic sublimation, iodine transitions directly from a solid to a beautiful violet gas when heated, bypassing the liquid phase. This characteristic makes iodine distinctly recognizable and fascinating to observe. The element emits a sharp, distinctive smell that is often associated with its antiseptic applications. In terms of solubility, iodine is moderately soluble in water, where it forms a brown solution, but it is highly soluble in nonpolar solvents like hexane, which can be used to extract iodine in its pure form. This solubility feature, combined with its volatility, underpins many of its uses in both scientific experiments and medical applications, where its antiseptic properties are highly valued.
Applications of Iodine in Science and Technology
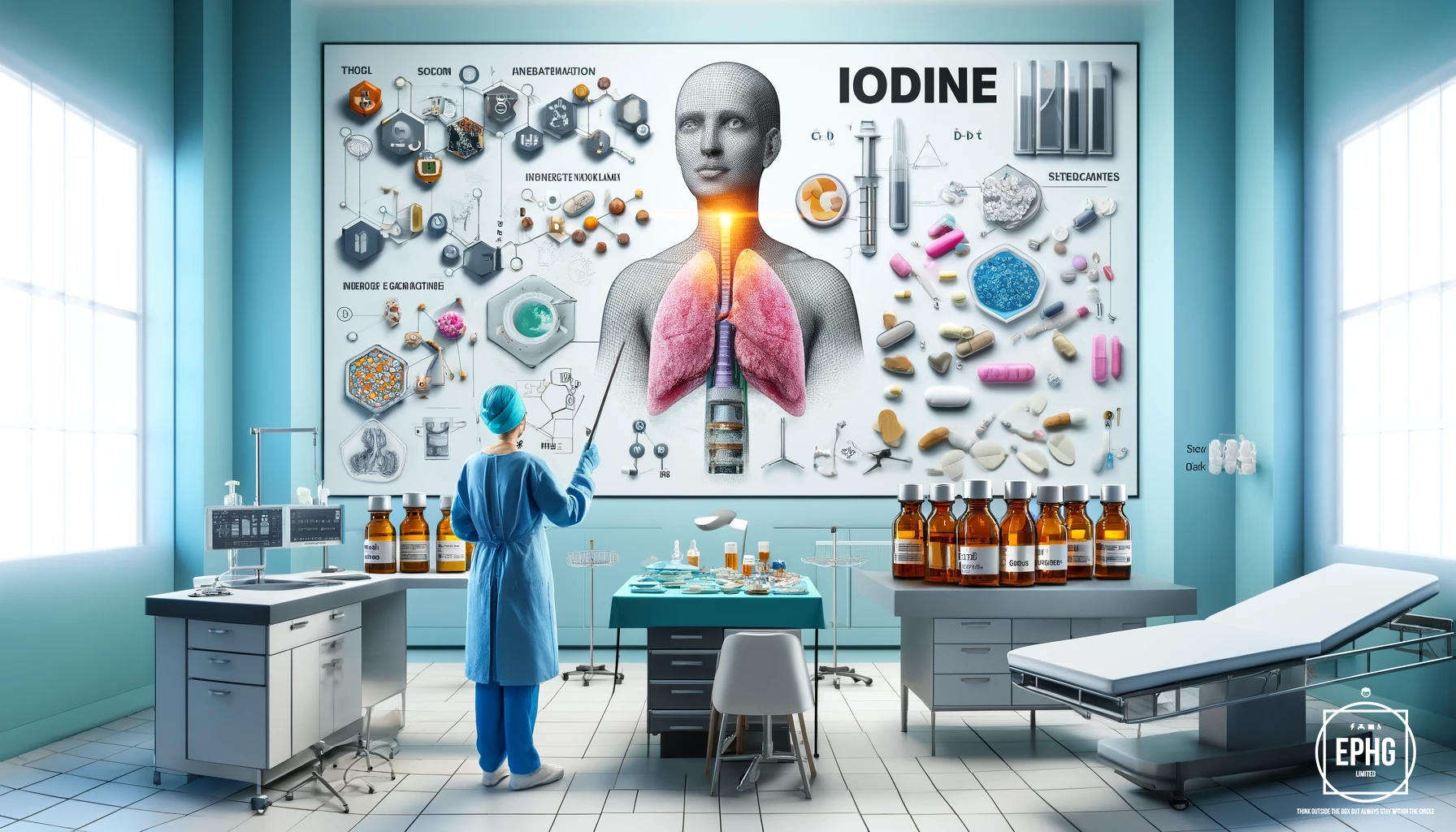
The applications of iodine are extensive and diverse, influencing multiple scientific and technological fields. In healthcare, iodine is indispensable for thyroid health as it is a fundamental component of thyroid hormones, crucial for regulating metabolic processes. Its potent antiseptic properties are leveraged in various medical settings, making iodine a primary ingredient in disinfectants and a range of pharmaceuticals aimed at preventing and treating infections.

In the realm of technology, iodine's role is equally significant. It is used in the manufacturing of polarizing filters for LCDs, enhancing the quality and performance of displays across devices. Iodine is also critical in the world of photography, particularly in the production of high-quality film where its light-sensitive properties are exploited to capture vivid images.
Moreover, iodine finds substantial use in chemical research, where its versatility as a reagent is invaluable. It participates in a wide array of synthetic processes, facilitating innovative reactions in organic chemistry. This capability allows for the creation of complex compounds essential in developing new drugs and materials, underlining iodine's integral role in advancing both science and technology.
The Role of Iodine in Global Health
Iodine deficiency is a major global health issue, particularly in areas where iodine-rich foods are scarce. Supplementation of iodine in salt has dramatically reduced conditions such as goiter and hypothyroidism in many regions. Ongoing research continues to explore the broader implications of iodine on cognitive development and overall health.
Future Prospects of Iodine
The future of iodine in science and technology looks promising. Researchers are exploring its potential in nanotechnology and advanced medicine, including cancer treatment and drug delivery systems. The unique properties of iodine may lead to new applications that could revolutionize how we treat diseases and manipulate materials at the molecular level.
How Iodine is Sourced

Iodine is primarily obtained from underground brines (water saturated with salt) where it is present as iodide salts. The largest producers of iodine today include Japan, Chile, and China. In Japan, iodine is extracted from seawater in coastal areas, where it is concentrated by natural processes. In Chile, iodine is mined from nitrate deposits where it coexists with other minerals like nitrate and sodium. The extraction involves pumping underground brine to the surface and using a method known as the blowout process, where iodine is liberated from the iodide with chlorine and then precipitated out to be further purified and processed.
Modern Uses of Iodine
In modern times, iodine continues to be a critical resource in medicine, nutrition, and industry. Its antiseptic properties make it a staple in surgical treatments and wound care products. Iodine is also vital in the global effort against iodine deficiency disorders, added to table salt to ensure adequate public intake. Industrially, iodine compounds are used in the manufacture of polarizing films for LCD displays and inks and colorants for photography and printing.
Futuristic Medical Treatment in Space

This image showcases a state-of-the-art medical treatment center aboard a starship, highlighting the advanced use of iodine-based technologies in a space setting. The medical bay is equipped with high-tech LCD cameras and digital displays, designed to monitor and treat patients with unprecedented precision. The futuristic medical staff, dressed in cutting-edge attire, are seen utilizing innovative iodine treatments to enhance patient care. The backdrop of outer space through large windows adds a profound sense of advancement and possibility, epitomizing the next frontier in medical technology.












